CAR T therapy is a revolutionary new cancer treatment that relies on engineering a patient’s immune cells. High transduction efficiency is key to its therapeutic success.
Nucleus Biologics has launched NB-ROC™, a serum free media formulated by T cell researchers with a focus on enhancing the critical quality attributes important to cell therapy developers. NB-ROC media will be a game changer for CAR T-cell therapy because it will significantly improve CAR T transduction efficiency, while preserving other T cell critical quality attributes.
What makes a T cell into a CAR T cell? The answer is the chimeric antigen receptor, or CAR, transduced into that cell to achieve a therapeutic effect. Cancer cells can evade immune detection through various mechanisms, including inducing the apoptotic cell death of cytotoxic T cells in the vicinity, and directly inducing T cell tolerance to cancerous cells.[1] CAR T cells can bypass this treatment hurdle because they are designed to express cell surface receptors that recognize specific antigens, (proteins), found almost exclusively on the surface of cancer cells. These antigen receptors are considered “chimeric” because they are not naturally expressed by a patient’s immune cells. Instead, they are genetically engineered receptors that are used to redirect T cells toward specific cancer cell surface markers such as CD19. CARs are introduced into the patient’s T cells through some form of delivery vector containing the genetic sequence for the receptor. Lentiviral vectors are commonly used as the CAR delivery system, since they provide a convenient method for gene transfer and permanent T cell modification.
Expression of a transduced CAR is what allows CAR T cells to directly target cancer cells. Thus, the higher the proportion of T cells successfully converted into CAR T cells via transduction, the greater the effectiveness of the CAR T treatment in killing off cancer cells.[2]
NB-ROC T cell media is formulated to be serum free, a decision made to eliminate the intrinsic variability that comes with media containing human serum. This, combined with the fact that the media is manufactured in an ISO 13485:2016-certified facility, help streamline regulatory compliance during development. But the benefits of NB-ROC don’t stop there. In a recent comparative study, Nucleus Biologics researchers evaluated the performance of T cells grown in NB-ROC and other medias. Cryopreserved PBMCs from 3 different healthy donors were used to isolate T cells. These cells were cultured using 3 different media combinations: an industry-leading T cell media (TCM) + 5% human serum, TCM + Physiologix™ media supplement, and NB-ROC + Physiologix. After 7 days in culture, T cells grown in each of the 3 T cell media combinations showed comparable viability, proliferation and population doubling rates.
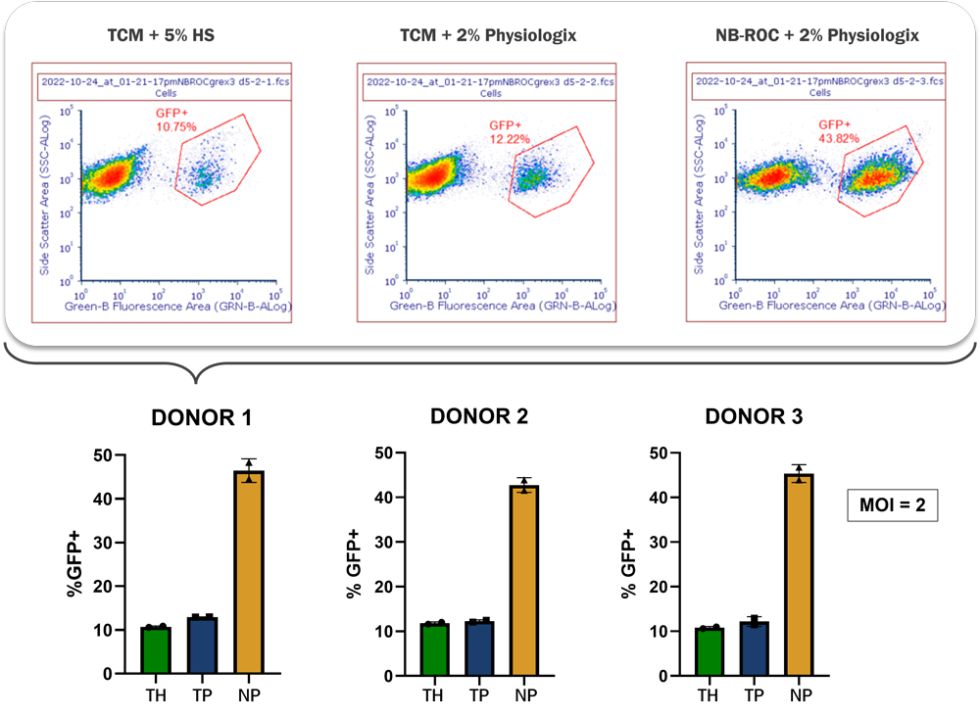
Next, the scientists measured lentiviral transduction efficiency, using GFP expression as a reporter gene for the experiment.
For all 3 donors, culturing T cells in NB-ROC + Physiologix resulted in a greater than 3-fold increase in GFP transduction efficiency, as measured by flow cytometry. Furthermore, cell count calculations revealed a low MOI (Multiplicity of infection) of 2, meaning that for T cells grown in NB-ROC + Physiologix, high transduction rates can be achieved using relatively low quantities of lentiviral vector, translating to a substantial cost savings.
Clinical trial results reported in the American Society of Hematology publication Blood Advances [2] reveal just how important CART T cell transduction efficiency is to patient outcomes. In that study, the CAR T therapy tisagenlecleucel was being evaluated for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) in young patients. Researchers used retrospective data to understand the effect of different CAR T cell doses on survival outcomes. 86% of patients who received the highest dose of CAR T cells within an approved range were alive one year after treatment versus 59% of those who received the lowest dose of that same range. The higher doses were not found to be associated with increased safety concerns.
This data eloquently drives home the real-world impact of optimizing critical quality attributes. NB-ROC T cell media results in more CAR positive cells than T cell media currently available, at a reduced cost, and while maintaining all other quality attributes. Lowering development costs helps to increase the number of therapies that go to clinical trial. Better CAR T transduction efficiencies means giving patients a better chance.
To learn more about NB-ROC and other Nucleus Biologics products, please visit our website today.
References
- Kim SK, Cho SW. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front Pharmacol. 2022 May 24; 13:868695. doi: 10.3389/fphar.2022.868695. PMID: 35685630; PMCID: PMC9171538.
- Higher CAR T Doses May Improve Survival Outcomes. ASH Clinical News. Nov 2022.
https://ashpublications.org/ashclinicalnews/news/6506/higher-car-t-doses-may-improve-survival-outcomes