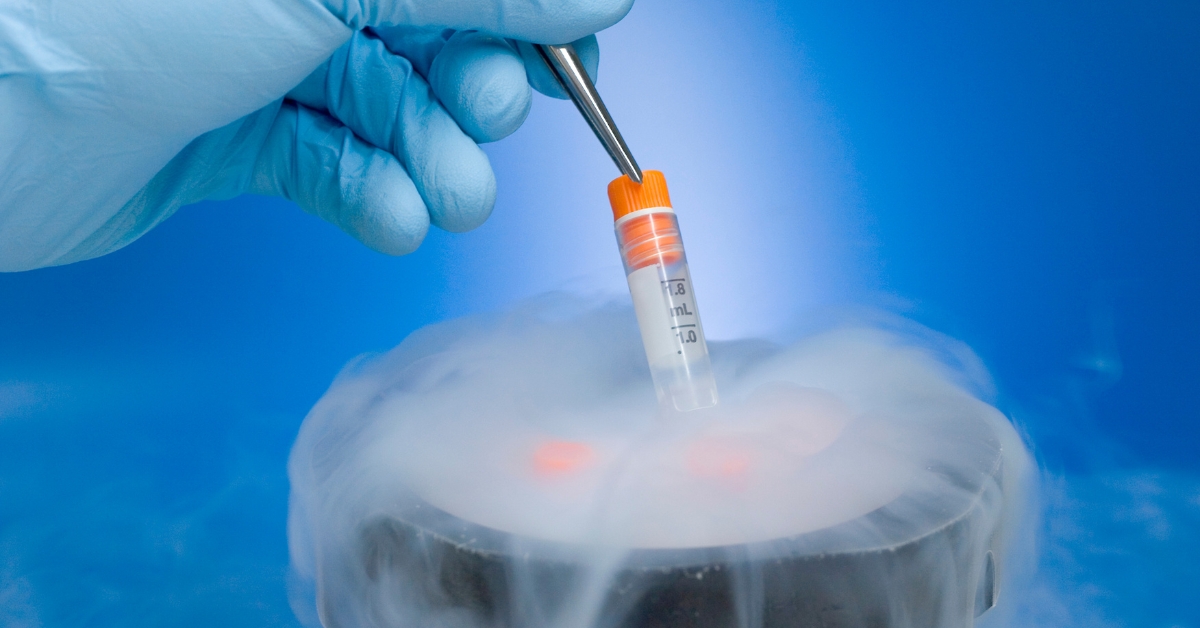
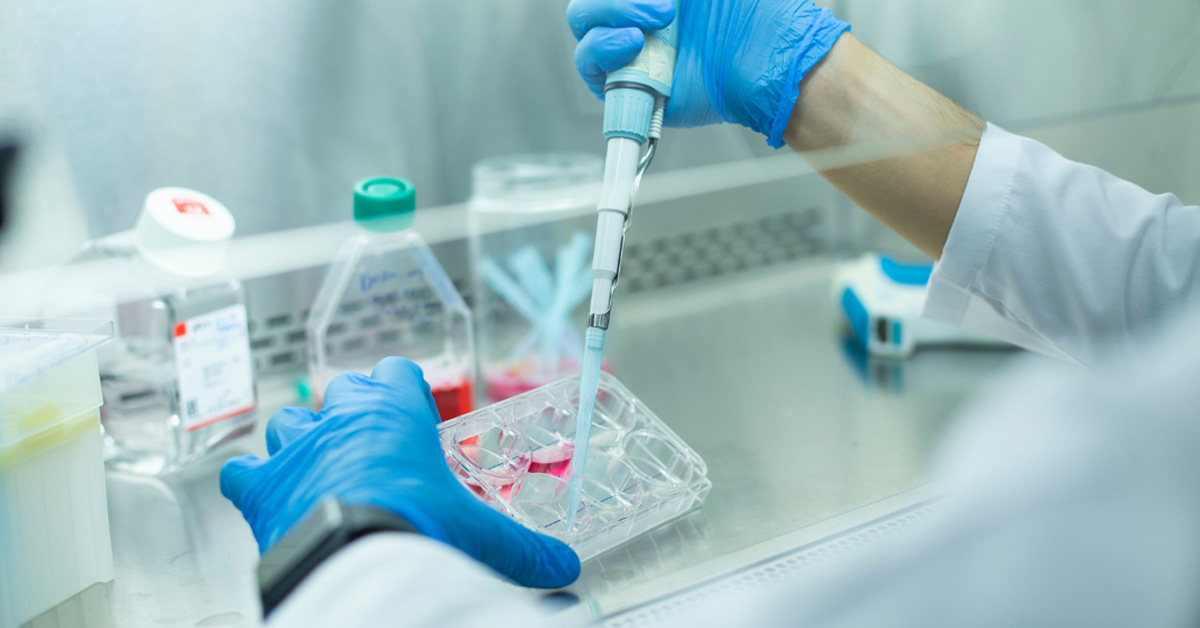
San Diego, CA – October 15, 2024 – Nucleus Biologics, a leading provider of cell culture and bioprocessing solutions for the cell and gene therapy (CGT) industry, has launched NB-KUL DF, a DMSO-free, chemically-defined cryomedia set to redefine cryopreservation standards. Designed for CGT manufacturers, NB-KUL DF matches DMSO-based media performance and outperforms other DMSO-free options in cell viability, recovery, and expansion. While DMSO is an effective cryoprotectant, its toxicity compromises cell viability, can cause patient issues, and requires complex wash steps, adding cost and variability. NB-KUL DF eliminates these issues, offering a DMSO-free solution that preserves cell integrity, eliminates the potential for patient side effects, and simplifies cryopreservation making it ideal for T cells, MSCs, and other sensitive cell types used in CGT.
Key Benefits of NB-KUL™ DF:
- Supports Viability and Recovery in Multiple Cell Types: NB-KUL DF ensures higher post-thaw viability and recovery than competitive cryomedia, optimizing therapeutic cell preservation for better clinical outcomes.
- Enhanced Expansion: Supports superior cell expansion when compared to competitive medias, meeting the demands of large-scale CGT manufacturing.
- DMSO-Free: Eliminating DMSO reduces cytotoxicity, protecting cells and patients, especially in high-dose or immunocompromised therapies.
- Streamlined Workflow: Simplifies processes by removing the need for DMSO washing steps, reducing cell loss, variability, time, and costs.
As part of Nucleus Biologics’ QuickStart Media™ platform, manufacturers can customize the product components, concentrations, packaging, and quality to meet their specific needs for optimized cell therapy production.
“The launch of NB-KUL DF marks a new era in cryopreservation of cells used for therapies,” said David Sheehan, CEO at Nucleus Biologics. “This product will reduce complexity and cost and eliminate patient side effects. The team at Nucleus Biologics continues to innovate to create GMP products that make these therapies more consistent and accessible to patients. With this launch and the customization capabilities, we are empowering CGT developers to optimize their processes while maintaining full control over product performance and quality. This ensures that every batch of cryopreserved cells is precisely what the customer and patient needs, when and how they need it. Simple is better.”
Nucleus Biologics is a pioneer in the development of innovative solutions for the cell and gene therapy industry. With a commitment to advancing the future of life-saving therapies, Nucleus Biologics delivers high-performance products that meet the complex needs of researchers and CGT developers worldwide. The launch of NB-KUL DF and its integration into the QuickStart Media platform reflect the company’s dedication to driving formulation transparency, safety, and scalability in the CGT space.
For more information about NB-KUL DF, the QuickStart Media platform, or how to customize your cryomedia solution, visit our website at www.nucleusbiologics.com.
About Nucleus Biologics
Nucleus Biologics is the leading provider of custom cell-growth media and buffer solutions, tools, and technologies for cell and gene therapy. Its mission is to speed the time from scientific discovery to cure by delivering innovative, transparent, and GMP products and services. From design to delivery, we offer an entire ecosystem of cell culture solutions that seamlessly interface with one another, facilitating easy formulation, configuration, ordering, and manufacture of media and buffers, while addressing the environmental impacts of cell culture fulfillment.
Media Contact:
Brad Taylor, Ph.D.
Vice President, Marketing
630-857-0556
[email protected]