Anyone who has ever handled human-induced pluripotent stem cells knows the challenges that arise during expansion. Safeguarding stem cell pluripotency can be tricky, particularly since fibroblast growth factor-2 (FGF-2), the primary growth factor that maintains the undifferentiated, pluripotent state, is thermally unstable and must be replenished daily.
Manufactured by Core Biogenesis, FGF-2 TOP is a ground-breaking, stabilized version of the growth factor that uses a novel ultra-scalable and sustainable plant-based bioproduction platform to offer a novel way to grow FGF-2-dependent cell cultures more efficiently, and with fewer media changes. The growth factor is available in the standard RUO format for testing and will soon be available in a GMP format as well by Nucleus Biologics.
Why Use FGF-2 TOP®?
- GMP quality available soon
- Significant cost advantages per vial
- Ultra-scalable
- 100% preservation of bioactivity
- Sustainable
- Thermostability saves time and money
An essential aspect of stem cell culture is the successful maintenance of the undifferentiated state. Many types of stem cells are FGF-2 dependent. The growth factor regulates a wide range of critical cellular functions including migration, proliferation, differentiation, and survival [1]. FGF-2 is therefore a necessary reagent in culturing protocols for iPSCs and is qualified for different applications including industrial cell-based meat production, cell culture, and cell therapy manufacturing.
Induced pluripotent stem cells (iPSCs) are maintained by replacing FGF2-containing media daily, while tissue-specific stem cells are typically fed every third day. Frequent feeding, however, results in significant variation in growth factor levels due to FGF-2 instability, which limits effective maintenance of the culture’s full pluripotent capacity because it allows for spontaneous differentiation.
To avoid loss of pluripotency, researchers and manufacturers have historically had to maintain a very strict daily cell feeding schedule. To streamline this time-consuming cell culture protocol and save on resources, scientists set out to create a more thermostable version of FGF-2 growth factor. Using computer-assisted engineering, a more stable variant was designed [2]. The result, FGF-2 TOP, is a recombinant 154 aa mature domain of FGF-2. The novel substitution of nine amino acids increases the functional half-life of the protein from <10 hours (wild-type) to >7 days (FGF-2 TOP) under standard cell culture conditions at 37ºC. FGF-2 TOP is derived from Core Biogenesis’ proprietary plant-based production system and is also fully animal component-free, endotoxin-free, and the product is sustainably produced.
By nature of its increased half-life and thermal stability, FGF-2 TOP exposes stem cells to a stable level of growth factor in culture, affording scientists a much more streamlined (and weekend-free) feeding schedule and ultimately a more homogenous makeup of the desired undifferentiated phenotype in the final stem cell population.
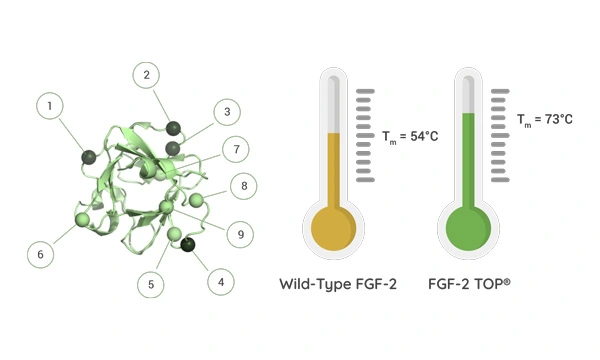
FGF-2 TOP preserves the full bioactivity and signaling required for FGF-2 mediated pathways, even after twenty days at 37°C. Researchers save valuable time and money because repeated supplementation with FGF-2 and a daily medium change are not required. Furthermore, the more stable presence of this critical growth factor results in an improved cellular proliferation profile compared to wild-type FGF-2.
What Sets Our FGF-2 TOP® Apart From the Crowd
FGF-2 TOP is currently available in Research Use Only format and will soon be available in a fully GMP-compliant format. The use of GMP reagents ensures that all regulatory protocols and SOPs are in place as developers scale up to a clinical setting. To ensure seamless scale-up, we have ensured that bioactivity and product performance of both grades are equivalent, as both grades are derived from the same molecule. The GMP version is manufactured in our GMP-compliant facility at Nucleus Biologics and is augmented with additional quality control testing and documentation to satisfy GMP cell culture requirements.
Like all Core Biogenesis products, FGF-2 TOP is produced using molecular farming – the production of recombinant proteins using the oilseed plant, Camelina sativa as an expression system. This technology offers several benefits compared to conventional protein expression methods. Expression yield is increased by orders of magnitude. Protein extraction is more efficient, easily scalable, and free from the risk of endotoxin contamination. Since expensive bioreactors are unnecessary, production is cost-effective and sustainable. None of the components or raw materials employed for the production of FGF-2 TOP are derived or extracted from animal or human origin.
The ongoing optimization of cell culture media and reagents will help to reduce costs and minimize risks across the cell and gene therapy industry. Nucleus Biologics is pleased to support this important work, and is offering RUO FGF-2 TOP samples to customers interested in trying the product in their own lab prior to purchasing the GMP-grade format.
To learn more about FGF-2 TOP RUO and GMP products, please visit our website.
References
- Chen G, Gulbranson DR, Yu P, Hou Z, Thomson JA. Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells. 2012;30(4):623-630. doi:10.1002/stem.1021
- Dvorak P, Bednar D, Vanacek P, et al. Computer-assisted engineering of hyperstable fibroblast growth factor 2. Biotechnol Bioeng. 2018;115(4):850-862. doi:10.1002/bit.26531




