It has been almost 7 years since the first CAR T therapy was FDA approved, and we are now beginning to incorporate lessons from first-generation commercialized therapies to make the next generation more scalable. The first approved therapies were autologous, so each lot was unique to the patient which resulted in high costs and limited scalability. Some valuable lessons were learned: individual components in your manufacturing process affect the therapeutic potency, closed systems are critical, and sole-sourced products are a risk both from a cost and supply standpoint. As a performance media and reagents supplier we saw this issue firsthand and launched a complete digital tool kit to 1) allow our customers to formulate their media based on critical quality attributes with our AI engine that searches peer-reviewed articles for component and concentration suggestions and 2) order cell culture products and store critical documents on an Amazon-like cloud-based portal. One limitation we recognized was that to truly scale up custom media, hit cost and time goals, and effectively eliminate human error from the manufacturing of media and reagents, we had to migrate to digital batch records. With formulation, customization, and manufacturing now feasible within one ecosystem, this represents what would be a digital custom cell culture media universe, or MediaVerse™. This sophisticated system was designed, built, and validated in-house which, as the first of its kind, will allow us to seamlessly integrate with our NB-Lux™ cloud-based ordering portal.
The Birth of MediaVerse™ – How the Digital Transformation Drove Its Inception
The digital transformation of the cell and gene therapy industry has officially begun, ushering in a new era of innovation and efficiency for the development of advanced therapeutics. As the convergence of cutting-edge biotechnology and digital technologies accelerates, industry stakeholders are increasingly turning to sophisticated digital solutions to revolutionize every aspect of the therapeutic lifecycle. Central to this transformation is the adoption of Electronic Batch Record (EBR) systems, which not only streamline manufacturing processes but also ensure compliance with stringent regulatory standards, notably Part 11 of Title 21 of the Code of Federal Regulations (CFR). This intersection of digitalization and regulatory compliance is poised to reshape the landscape of cell and gene therapy, propelling the industry towards greater reliability, scalability, and ultimately, more accessible treatments for patients worldwide.
An EBR is a digital system used in manufacturing processes to document and manage batch data electronically. It essentially replaces the traditional paper-based method of recording manufacturing data, allowing for more efficient, accurate, and compliant documentation. EBR systems typically include features such as electronic signatures, version control, and audit trails.
One critical aspect of EBR systems is ensuring compliance with regulatory standards, particularly Part 11 of Title 21 of the Code of Federal Regulations (CFR). Part 11 sets forth the criteria under which electronic records and signatures are considered trustworthy, reliable, and equivalent to paper records and handwritten signatures. Compliance with Part 11 involves implementing controls to ensure the security, integrity, and authenticity of electronic records, as well as implementing procedures for electronic signatures.
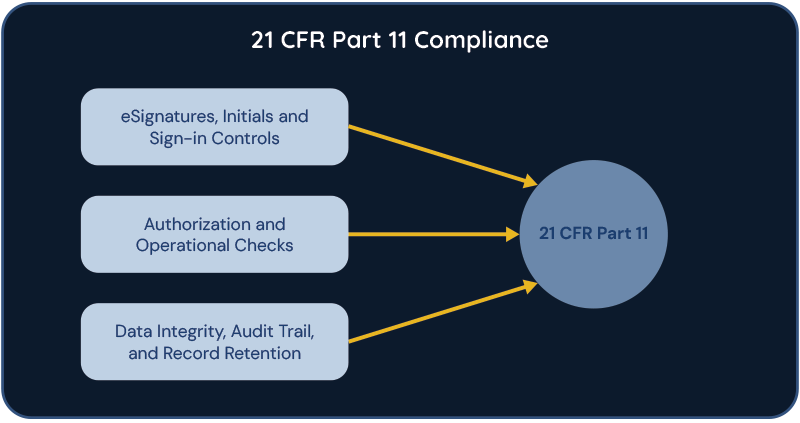
For the cell and gene therapy industry, EBR systems and Part 11 compliance are critical due to the highly regulated nature of the field. Cell and gene therapies involve complex manufacturing processes that require meticulous documentation to ensure product quality, safety, and efficacy. EBR systems streamline these processes by providing a centralized platform for recording and managing batch data, facilitating real-time monitoring and analysis, and enabling rapid decision-making. Moreover, compliance with Part 11 ensures that electronic records and signatures generated by EBR systems are legally and regulatory accepted. This is crucial for demonstrating the traceability and accountability of manufacturing processes, as well as for achieving regulatory approvals from agencies such as the Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Ultimately, EBR systems and Part 11 compliance play a critical role in advancing the development and commercialization of cell and gene therapies by ensuring quality, compliance, and patient safety throughout the manufacturing lifecycle.
The Inception of MediaVerse™
The reliance on manual methods for documentation and traceability in our current cell culture media manufacturing processes underscores the critical need for digital transformation within our operations. While manual methods have sufficed quite well in the past, the complexity and scale of our manufacturing operations today demand a more sophisticated approach. Manual documentation not only introduces the risk of human error but also hampers efficiency and scalability. The lack of real-time data access and centralized record-keeping impedes decision-making processes and hinders our ability to track and analyze critical manufacturing parameters. Transitioning to an EBR system represents a pivotal step towards modernizing our manufacturing practices, enabling us to enhance traceability, streamline documentation processes, and ultimately, optimize the quality and efficiency of our cell culture media production.
The quest for an EBR system tailored to the unique demands of cell culture media manufacturing presents a formidable challenge within the biopharmaceutical industry. Unlike traditional pharmaceutical manufacturing, cell culture media production involves intricate formulations and highly variable raw materials, necessitating a flexible and adaptable EBR solution. Finding a system that seamlessly integrates with the complex processes inherent to cell culture media manufacturing, while also ensuring compliance with regulatory requirements such as Good Manufacturing Practices (GMP), can be daunting. Moreover, the dynamic nature of cell culture media production, with its frequent formulation changes and batch variations, further complicates the selection process. Balancing the need for comprehensive data capture, real-time monitoring, and scalability with the intricacies of cell culture media manufacturing presents a significant hurdle that requires careful consideration and evaluation of available EBR options. The difficulty of identifying a system that would meet Nucleus Biologics’ stringent requirements prompted the design and development of our internal platform MediaVerse.
How We Built MediaVerse™
In order to design a system that would meet the needs of our demanding media manufacturing process, we performed both a thorough voice of internal customer as well as a comprehensive risk assessment and identified some key areas for improvement including goals to significantly decrease production time (>40%), reduce batch record flags and NCRs by >90%, as well as implementing steps to create a 21 CFR Part 11 compliant environment. As mentioned, EBRs provide traceability and quality for even the most stringent of client requirements. With MediaVerse, we will be able to provide products with improved lead times, quicker QA turnaround, and superior traceability.
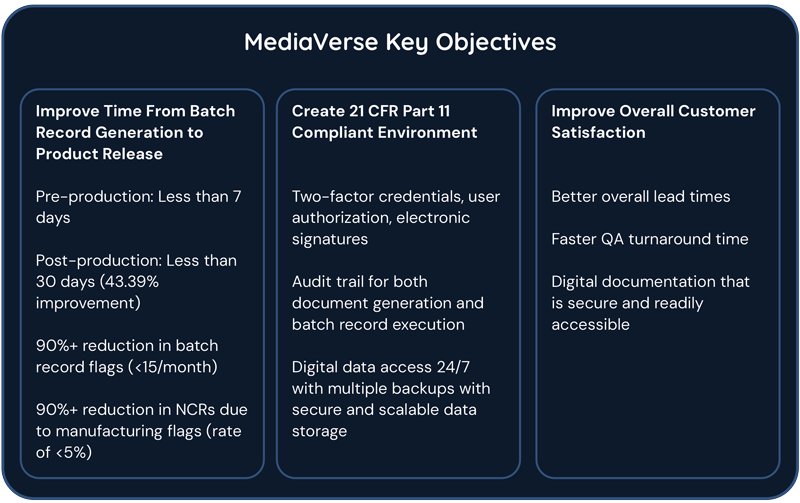
Incorporating MediaVerse will usher in a new era of efficiency and transparency in our manufacturing processes, significantly enhancing the quality and productivity of our operations while bolstering traceability throughout the lifecycle. By digitizing our batch records, we will have streamlined data entry and retrieval, minimizing errors and delays associated with manual documentation. This heightened efficiency not only accelerates the pace of production but also fosters a more transparent environment, where stakeholders can access real-time information on manufacturing activities. Moreover, the implementation of MediaVerse enables us to uphold higher levels of quality assurance by enforcing standardized processes and ensuring compliance with regulatory standards. As a result, our organization will experience a notable increase in productivity, coupled with a strengthened ability to trace and analyze data, ultimately driving continuous improvement and innovation in our pursuit of delivering safe and effective cell and gene therapies.
In conclusion, the implementation of MediaVerse marks a transformative leap forward for Nucleus Biologics and its commitment to excellence in cell culture media manufacturing. By streamlining processes through electronic batch records, we are not only enhancing lead times but also elevating the quality of our products to unprecedented levels. The enhanced traceability afforded by MediaVerse ensures complete transparency and accountability throughout the production lifecycle, bolstering regulatory compliance and reinforcing customer trust. Ultimately, the overarching impact of this innovative system extends far beyond our internal operations, resonating profoundly with our valued customers by delivering unparalleled service and satisfaction. With MediaVerse, Nucleus Biologics is poised to lead the industry into a new era of efficiency, reliability, and customer-centricity.
Contact us today to discover how we can help speed your discovery to cure.




