Nucleus Biologics, together with its spinoff company Stoic Bio, is reimagining cell culture media manufacturing to bring the pharmaceutical industry a system that can deliver custom media in a sustainable manner-all while lowering the total delivered cost.
In our previous blog, we talked about scalability and how to simplify the transition to large-scale media production. This week, we bring you the fourth pillar upholding the Krakatoa™ cell culture media system: Lower Total Delivered Cost.
While most researchers would love the opportunity to have custom designed cell culture media delivered fresh to their lab, the resources involved can be an impediment. Since changing even a single component of cell culture media can impact cell health and performance, it’s important to get things right. But the process of researching, configuring, and testing custom media is time-consuming and expensive.
Cost is a critical concern for cell therapy developers for several reasons. The total delivered cost of a therapeutic is a primary driver of how competitive it is compared to similar or alternative treatments. [1, 2] Cost significantly impacts how much of the pharmaceutical market share the final product is likely to garner, how many patients will be able to benefit from the treatment, and ultimately, whether a product will be considered a viable candidate for development in the first place.
Cell culture media is a major cost driver in the cell and gene therapy industry. Krakatoa and the suite of online tools backing it can help companies significantly lower development costs for cellular therapeutics, particularly during scale-up, an inevitably sensitive inflection point in the transition from preclinical to commercial production.
Unlike conventional cell culture media, Krakatoa media (whether traditional or custom) is manufactured at the point of use. With cloud-based customization, ordering, and tracking, media formulation is rapid and user-friendly. According to in-house calculations, Nucleus Biologics’ media configuration tools NB-Lux™ and NB-AIR™ save over 11 months of research and development time when compared to traditional custom media development and delivery timelines, and more than three weeks of development time even if proprietary off-the-shelf media formulations are used. Additionally, Krakatoa media is delivered in lightweight, biodegradable pods that can be shipped and stored at ambient temperatures. This model results in a huge savings in single-use plastics, packaging, shipping weight, waste generation, and cold chain related energy consumption.
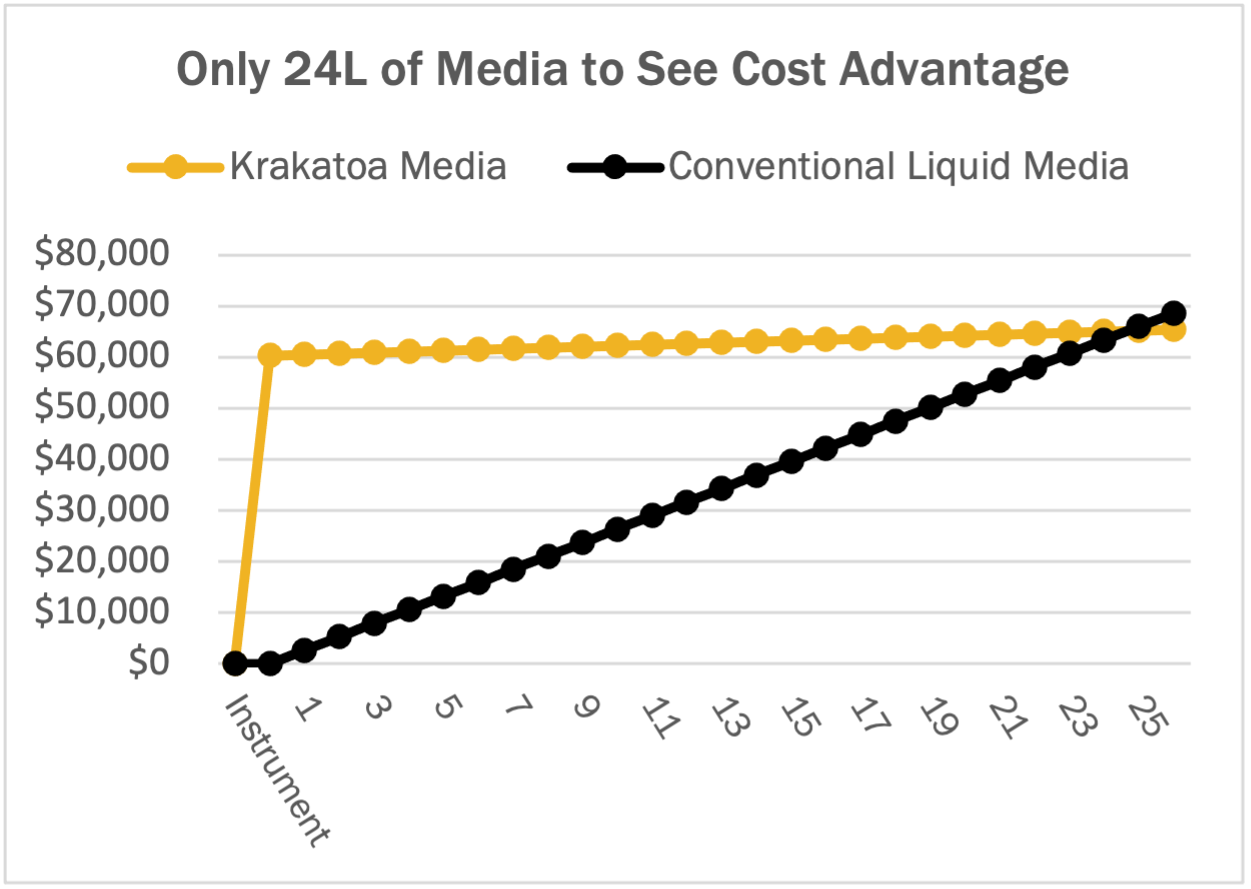
Krakatoa is designed to support fast, small-batch custom media iteration and testing. Although small volume orders of < 2L custom media can still be slightly more expensive per liter than conventional liquid media, Krakatoa’s minimum order volume of only 500 mL, allows customers to test formulations at smaller volumes, reducing media waste, and ultimately, total cost. At the 1-10L minimum order volumes customary to competing media suppliers, the cost savings soon become evident. As order volumes ramp up, prices are actually less expensive than conventional media, and downstream ordering and scale up steps are simpler.
When comparing total delivered costs, Krakatoa pods are, on average, 1/10th the cost of conventional liquid custom media. Even considering the initial instrument cost, users can realize cost benefits in the first year, if only 4 pods of custom media are run per month. After ordering just 48 pods, users will completely recoup the cost of the instrument. Since overhead and maintenance costs drop in the second year, Krakatoa users will realize even more cost advantages in subsequent years of use.
Once users have perfected their media formulation in small volumes using Krakatoa, scaling up using the Nucleus Biologics ecosystem continues to provide cost benefits. To provide a conceptual example of cost savings, Nucleus Biologics performed computations for cell culture media purchased at the volume and pricing representative of commercial-scale production. Assuming a company is manufacturing a T cell therapy for 5000 patients per year, the equivalent liquid media consumption would be approximately 14L per patient. The average cost for liquid media delivery of proprietary off-the-shelf T cell media with standard supplements, at volume, is $299.50 per liter. Meanwhile, the cost of custom media formulation using Nucleus’ suite of tools for fulfillment of the same volume is $189.00 per liter, or a 37% savings. This equates to a savings of $8 million per year for this company. Furthermore, by using the Nucleus Biologics media formulation tools, the researcher gains complete transparency regarding media components, owns the intellectual property of their formula, and has only 1 vendor to qualify and one process to validate for regulatory purposes.
One can see how these cost savings quickly add up, but there are other benefits to using the Krakatoa on-site media manufacturing model. Liquid media degrades over time, with key amino acids and vitamins breaking down even in cold storage. This can lead to increased waste in the form of expired media, or poor cell performance when media is not at its peak. Due to the weight of liquid media, international shipping costs can amount to more than the value of the media itself, and cold chain disruptions can result in loss of media quality.
Powder media pods are small and lightweight. They can be stored on-site at ambient temperature, lowering supply chain risks and reducing energy consumption. The Krakatoa system primarily uses biodegradable and reusable materials, greatly reducing the lab’s environmental footprint.
All of these considerations are important when developing a cell therapy. However, perhaps the most important point is that lower total delivered cost makes it more likely a cell therapy will be developed. Development costs are by necessity passed on to patients—lower total delivered cost ultimately equates to better patient access, a win for all of us.
To learn more about how the Krakatoa media manufacturing system can help accelerate your research program, please visit the Nucleus Biologics website today!
References
- Hildreth C. The Cost of Stem Cell Therapy in 2022. BioInformant. February 2022. https://bioinformant.com/cost-of-stem-cell-therapy/
- Renske MT et al., What does cell therapy manufacturing cost? A framework and methodology to facilitate academic and other small-scale cell therapy manufacturing costings.




