Recombinant Human FGF-2 TOP® GMP
FGF-2 TOP GMP is a thermostabilized, recombinant human growth factor manufactured in a novel plant-based expression system that provides a cost-effective, GMP-quality solution for the growth of stem cells and adherent HEK cells.

The FGF-2 TOP® GMP Advantage

Plant-Based and Sustainable

Significant Cost Savings

Scale at GMP Quality
A Convenient Alternative with Cost Advantages
In order to maintain pluripotency and avoid spontaneous differentiation of stem cells, scientists traditionally had to maintain a strict daily feeding schedule due to the short half-life (approximately 9 hours at 37°C) and temperature sensitivity of wild-type FGF-2.
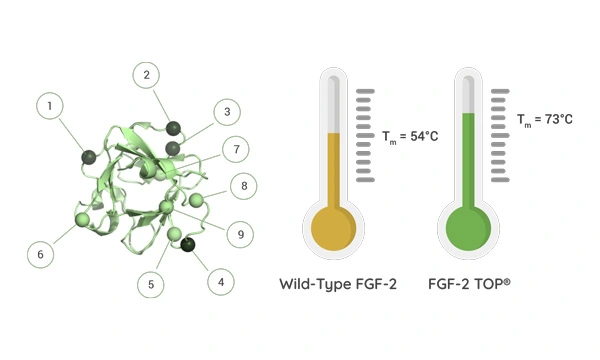
10-Fold Improvements in Half-Life at 37°C
To improve stability, a novel nine amino acid substitution of the wild-type FGF-2 was performed, improving the heat stability of FGF-2 TOP resulting in an increase in half-life under cell culture conditions to 37°C.
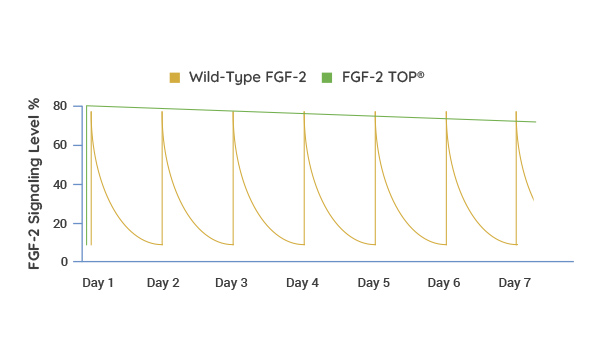
Supplement Less and Achieve More Consistency
By nature of its increased half-life and stability, FGF-2 TOP presents a constant exposure of growth factor (green line) in contrast to the short half-life and signaling of the wild-type protein that must be replenished daily (yellow line). Thus, feeding schedules are more streamlined and cell culture phenotype is more homogenous.
The Stability to Support Your Development and Scale-Up
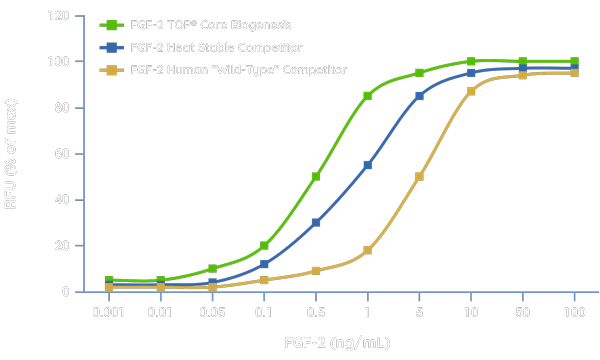
Preservation of Bioactivity
Comparison of FGF-2 TOP with a FGF-2 heat stable competitor and human FGF-2 wild-type in a 3T3 cell proliferation assay using varying concentrations of each protein after a 48-hour incubation at 37°C. FGF-2 TOP demonstrates maintenance of full bioactivity and with a 5-fold lower EC50, FGF-2 TOP demonstrates a greater capacity to promote 3T3 cell proliferation and at lower concentrations than competing heat stable alternatives and wild-type FGF-2 in this model.
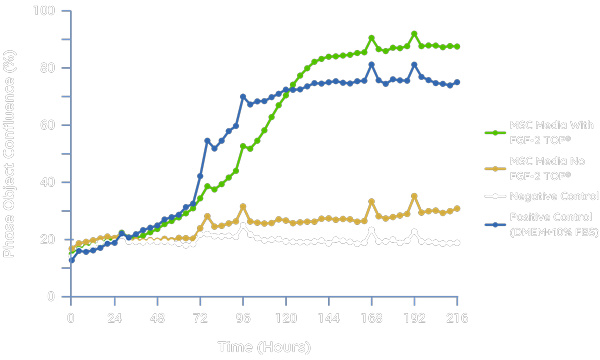
Supporting Proliferation of Adherent Cell Culture
In an internal MSC model analyzing confluence over 9 days, the addition of FGF-2 TOP to an internal MSC formulation resulted in an equivalent cell confluence and expansion (as assessed through cell attachment) compared to the positive control containing 10% FBS. Similar results have been observed in both iPSC and adherent HEK-293 models (assessed but not shown).
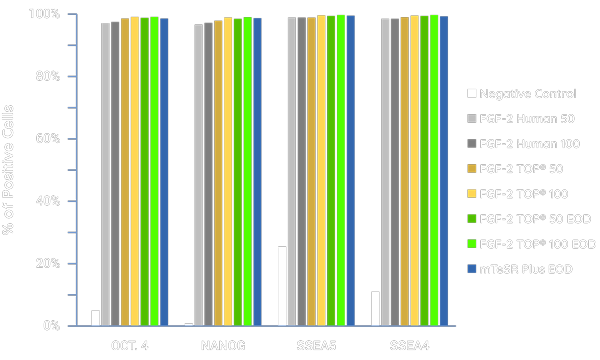
Maintains Pluripotency and Stemness
FGF-2 TOP maintains high-quality iPSC cultures under various concentrations and media feeding regimes as assessed by flow cytometry. Cells were treated with wild-type “human” FGF-2 or FGF-2 TOP at 50 or 100 ng/mL either daily if not indicated or every other day (EOD). The negative control contained no FGF-2 supplementation. All tested conditions resulted in high expression of pluripotency markers. Most notably, EOD feeding with FGF-2 TOP showed equivalent levels as well as provided further proof of bioactivity maintenance after stability changes. Source: App Note: Core Biogenesis in collaboration with TreeFrog Therapeutics.
Recombinant FGF-2 TOP®
FGF-2 TOP® RUO Sample
Catalog number: 2009
50 µg
$300

FGF-2 TOP® RUO Sample
Catalog number: 2040
1 mg
$900
FGF-2 TOP® GMP
Arriving early 2025
50 µg
Inquire for price
To ensure seamless scale-up, bioactivity and product performance of RUO- and GMP-grade will be equivalent, as both grades are derived from the same molecule.

FGF-2 TOP® GMP
Arriving early 2025
1 mg
Inquire for price
To ensure seamless scale-up, bioactivity and product performance of RUO- and GMP-grade will be equivalent, as both grades are derived from the same molecule.
Specifications
| Type | Recombinant protein |
| Amino Acid Sequence | 154 amino acids with 9 aa point mutations from the wild-type human FGF-2. Tag-free. Original reference sequence accession number: P09038 |
| Origin | Plant-derived; FGF-2 is produced in plant seeds of Camelina sativa. |
| Quality | RUO and GMP |
| Identity | Molecular weight via SDS-PAGE |
| Bioactivity | EC50 |
| Purity | ≥ 95% |
| Concentration | 1 mg/mL |
| Mycoplasma | RUO: No GMP: PCR |
| Bioburden | RUO: < 10 CFU/mL GMP: USP <61> |
| Endotoxin | RUO: < 100 EU/mL GMP: USP <85> ≤ 50 EU/mL |
| Shelf Life | Stability study in progress |
| Release | RUO: COT GMP: COA |
| Shipping Conditions | ≤ -10°C |
| Storage Temperature | -80°C |
| Typical Applications | iPSCs, organoids production, MSCs, HEK adherent |

Scalable, Consistent Plant-Based Bioproduction
Core Biogenesis produces FGF-2 TOP from Camelina sativa seeds, engineered for high-yield expression of recombinant growth factors. Cultivated in controlled chambers or open fields, this process offers scalable, cost-effective, and carbon-negative production. Seed yield and storage provide a sustainable solution for supply chain consistency. FGF-2 TOP GMP is manufactured at Nucleus Biologics’ GMP facility, under ISO 13485:2016-certified quality standards.
Documents and Downloads
Take a closer look at our helpful product information to learn how our cell culture media products and services can support and accelerate your groundbreaking research.
Review the technical details, features, and benefits of recombinant FGF-2 TOP.
Need Help?
Get in touch with our customer support team for any questions you may have.
Visit NB-Lux™
Formulation Services
Leverage our experts to develop or optimize your media.
Resources
Explore our educational resources to learn how Physiologix can be a competitive advantage on your path to commercialization.
FAQs
Is it possible to request a sample?
Yes! If you wish to request a sample, click here to fill out our request form.
Is FGF-2 TOP® GMP a Nucleus Biologics product?
FGF-2 TOP is produced by Core Biogenesis. In 2024, Nucleus Biologics and Core Biogenesis announced a strategic collaboration for the manufacture and distribution of FGF-2 TOP GMP by Nucleus Biologics. FGF-2 TOP can be purchased through Nucleus Biologics.
Can I use Recombinant FGF-2 TOP® GMP in my custom media?
Yes! FGF-2 TOP can be included as a custom component in your media formulation.
How is the quality control for Core Biogenesis plant-derived growth factors?
Core Biogenesis offers its customers effective, pure, and safe products. They carry out rigorous analysis of their products, including purity assessment to assure customers high-quality products. Growth factors are tested for biological activity by independent third-party companies or laboratories, and this information is provided in a data sheet for each batch of every product.
For how long are Core Biogenesis growth factors stable?
The QC testing backing our products has assessed to date, full performance and maintenance of the stated bioactivity for up to 6 months since product release. Core Biogenesis is currently determining the full shelf-life of our products before and after reconstitution for up to 2 years. Core Biogenesis believes in total transparency about their products and data.