Cell and gene therapy products are only as good as their cellular building blocks— therefore cell culture media should first and foremost be designed to support consistent and optimal cellular performance.
Welcome to Part 2 and the final part of our blog series highlighting PhysiologixTM — our cGMP, xeno-free media supplement. In Part 1 of this series, we discussed how variability in raw material sourcing can be mitigated with Physiologix. This week, we will examine how Physiologix delivers optimal cellular performance.
When cell therapy developers are sourcing raw materials for their therapeutic, the primary concern is likely to be which supplier best fits their needs. Is GMP-compliant material available? How consistent is the source material? Can the material scale with my therapeutic development? These questions are critical to streamlining the development process. However, another very important question to ask concerns optimization of performance.
Cell culture media composition and reproducibility can have beneficial impacts on the functionality of the final product. Cell health is of course an obvious goal when choosing culture media, but developers should also be thinking about cellular performance as a criteria in selection of their cell media. A successful therapeutic must demonstrate both efficacy and consistency. This can be a difficult task to achieve when therapeutics are based on living cells. Cellular products are prone to variability because cells are capable of responding to even minute changes in their environment. The task of the ideal cell culture media developer is thus to create a product designed to reduce variability in cell culture conditions while promoting optimal therapeutic functionality.
Physiologix is designed to support critical quality attributes (CQAs) of therapeutic cell types
Physiologix is formulated using a proprietary GMP manufacturing process and rigorous quality controls that ensure reproducibility of the product. As demonstrated in the previous blog, lot-to-lot consistency is validated.. Furthermore, Physiologix is specifically designed to support several CQAs beneficial in the development of cell and gene therapies, namely transduction efficiency and phenotypic maintenance.
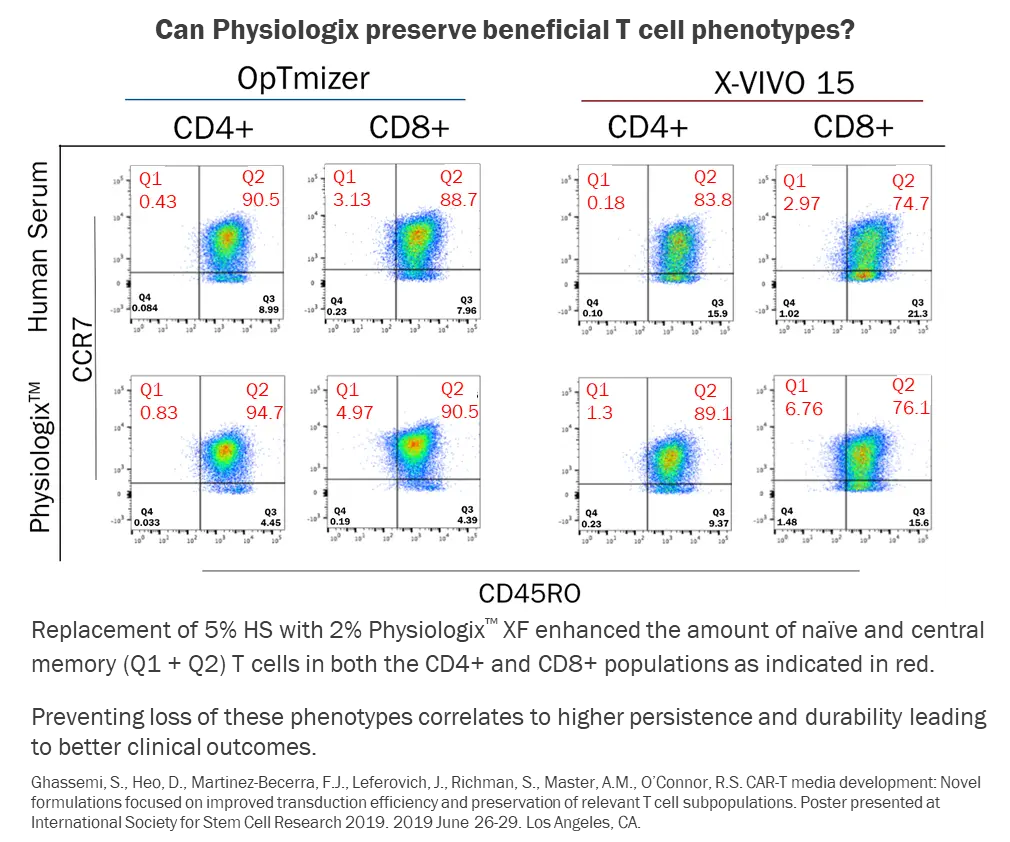
A comprehensive understanding of how each distinct media component affects living cells is critical to formulating an effective media supplement. Media and supplement formulation should be designed to enhance the functionality of the final product. CAR-T cell therapy, for example, is based on T cell function. More specifically, however, it is based on the enhanced presence of naïve and central memory T cell phenotypes. These phenotypes correlate with higher T cell persistence and durability, which contributes to better therapeutic efficacy and clinical outcomes. Physiologix has demonstrated enhanced preservation of relevant phenotypes compared to human serum supplementation. Physiologix supplementation in various basal media enhanced the number of naïve and central memory T cells in both CD4+ and CD8+ populations. Additionally, Physiologix can be used at lower concentrations than human serum to achieve better phenotypic results and deliver more optimal cellular performance.
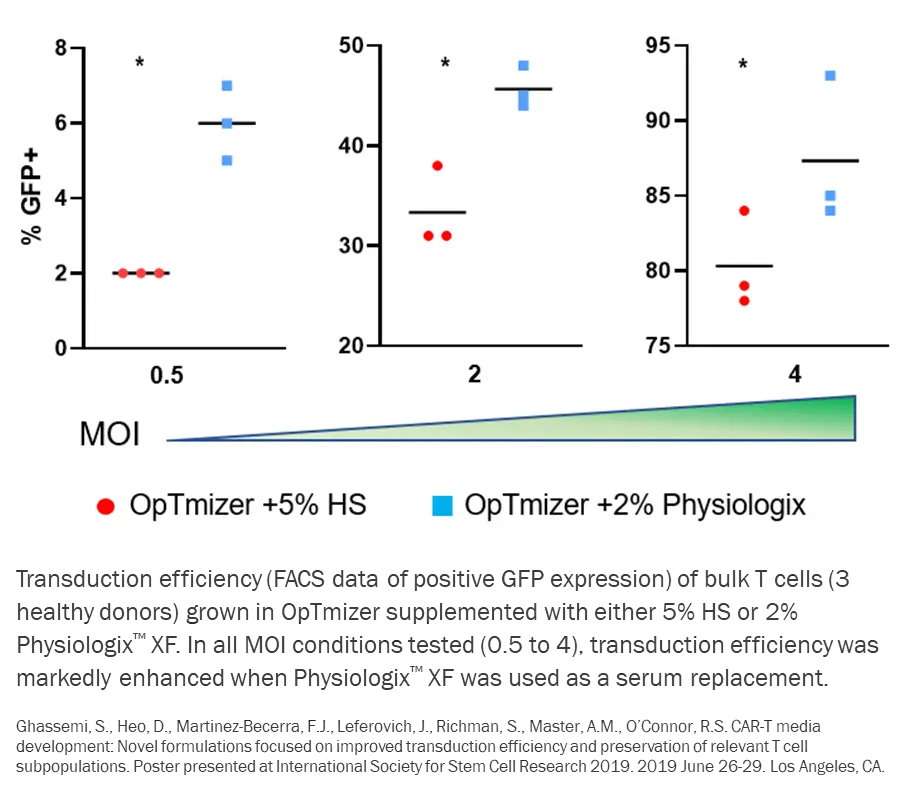
The potency of CAR-T cell therapies is directly related to the transduction efficiency of the CAR product. Physiologix’s formula contains physiologically optimized growth factors that promote viral entry and enhance lentiviral-mediated gene expression. Media supplemented with Physiologix has been proven to enhance transduction efficiency relative to human serum at both low and high viral titers, thus increasing the proportion of CAR transduced T cells in the final product.
Superior products give superior results
Optimal cell culture conditions affect the efficacy of the final therapeutic product. Once a cell-based therapeutic is infused into a patient, every healthy, functional therapeutic cell increases the chance of a successful outcome.
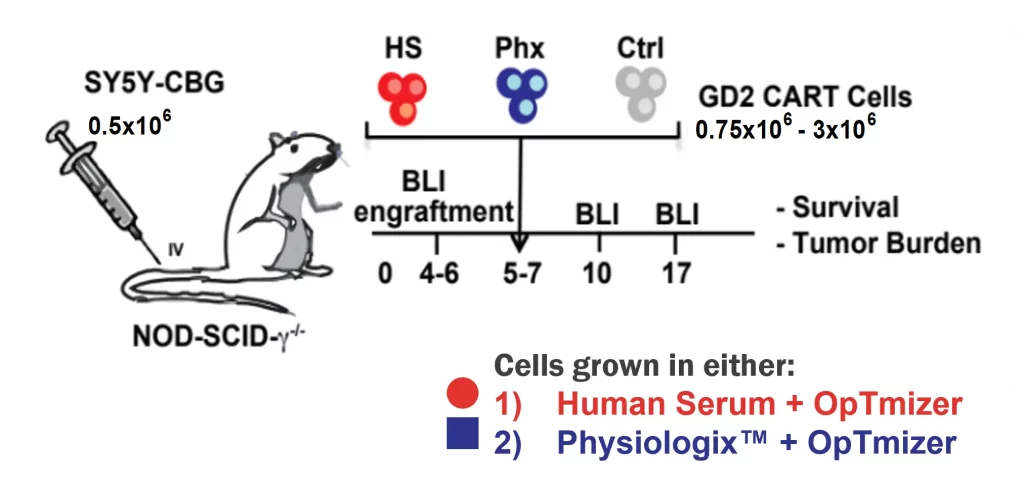
When developing and manufacturing a CAR-T cell product, the most important CQA is cytotoxicity— how well and for how long the product kills cancer cells. Physiologix has been proven to improve the therapeutic potential and in vivo efficacy of CAR-T cell therapy by promoting a more potent antitumor response of the CAR-T cells following transplantation in solid tumor mouse models.
In a recent publication [1] from the University of Pennsylvania, switching from human serum media supplement to Physiologix resulted in significant therapeutic benefits. In a xenograft mouse model of neuroblastoma, T cells expanded in Physiologix had superior engraftment in vivo compared to T cells expanded in human serum. This ultimately resulted in a logarithmic reduction in tumor burden.
Molecular Therapy, Methods and Clinical Development, VOLUME 18, P595-606, SEPTEMBER 11, 2020; Enhancing Chimeric Antigen Receptor T Cell Anti-tumor Function through Advanced Media Design; Saba Ghassemi, Francisco J. Martinez-Becerra, Alyssa M. Master, Sarah A. Richman, David Heo, John Leferovich, Yitao Tu, Juan Carlos García-Cañaveras, Asma Ayari, Yinan Lu, Ai Wang, Joshua D. Rabinowitz, Michael C. Milone, Carl H. June, Roddy S. O’Connor
At Nucleus Biologics, we understand that healthy cells perform better. Strategic design of cell culture media and supplements can be used to enhance the critical quality attributes needed for your therapeutic to succeed. Please visit our website to learn more about our formulation services, products, and how Physiologix delivers optimal cellular performance.
References
Gottlieb S. Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies. U.S. Food and Drug Administration. (2019).