Physiologix™ XF SR

The Physiologix™ Advantage

Streamline Regulatory Compliance
GMP and completely xeno-free, Physiologix can simplify your transition from development to commercialization.

Proven Serum Replacement
Drive desirable CQAs at superior or equivalent levels to FBS and human serum in both T cells and stem cells.

Consistent Quality
Proprietary manufacturing process and rigorous quality control yield documented consistency across lots.
Take Your Cells Farther
Replacing serum with xeno-free, GMP Physiologix brings consistent quality, increases cell performance, and streamlines regulatory to your cell culture research.
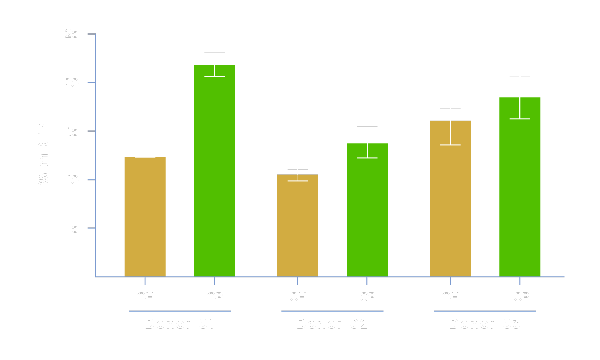
Enhances Transduction Efficiency
Physiologix promotes viral entry and enhances lentiviral-mediated gene expression. As shown, media supplemented with 2% Physiologix (OP) demonstrate significantly enhanced transduction efficiency measured as % expression of red fluorescent protein (RFP) (compared to 5% human serum (OH)), resulting in a greater proportion of transfected cells and, in turn, a higher potency final product at lower manufacturing costs. Data shown represents 3 donors using Optimizer™ media in a G-Rex® model.
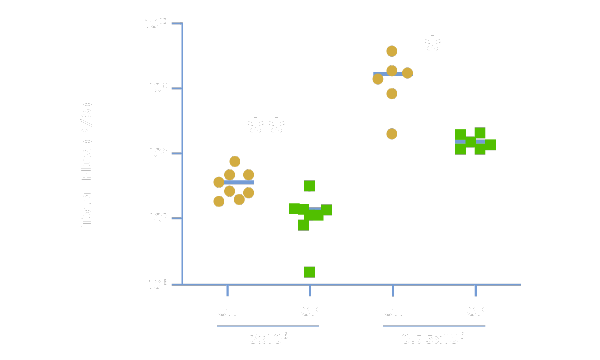
Greater Efficacy With More CAR+ Cells
Physiologix can improve the therapeutic potential and in-vivo efficacy of CAR T-cell therapy by promoting a more potent antitumor response. In data published by UPenn and Nucleus Biologics, anti-GD2 CAR-T cells expanded in OpTmizer™ + 2% Physiologix (OP) demonstrated significantly superior tumor killing in an in-vivo solid tumor neuroblastoma model when compared to CAR-T cells grown in OpTmizer + 5% human serum (OH). Data shows quantification of tumor burden in total flux (photons/second) by bioluminescence imaging on days 15 (3×10⁶ CAR-T cell dose) and 19 (0.75×10⁶ CAR-T cell dose).
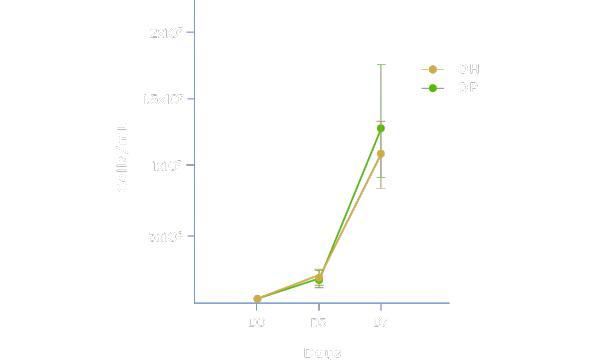
Maintains Cell Health and Desirable Characteristics
Physiologix maintains cell health, viability, and desired phenotypes equivalent to human serum. Media supplemented with 2% Physiologix (OP) in a 7-day analysis in a G-Rex® model demonstrated strong proliferation and population doubling (data not shown) when compared to media + 5% human serum (OH). Additionally, desired naïve and central memory phenotypes are preferentially maintained as with human serum. Data shown represents average of 3 donors ±SD using Optimizer™ media.
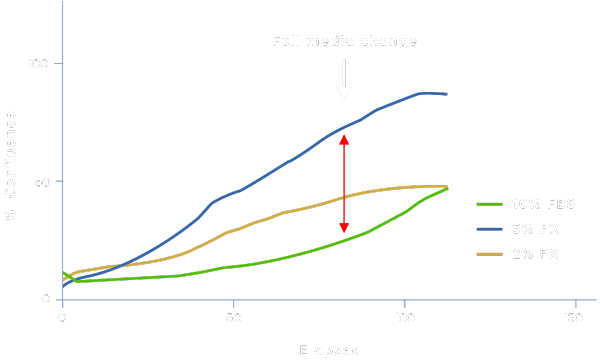
Applicability in Stem Cell Models
Physiologix can support proliferation in other cell models such as mesenchymal stem cells (MSCs). DMEM/F-12 media supplemented with Physiologix (PX) have increased cell proliferation rates at earlier time points (compared to FBS) resulting in shortened protocols, which could reduce labor and consumable costs, lower energy consumption, and accelerate therapy delivery without affecting the quality of the cells.
Physiologix™ Human Growth Factor Concentrate

Physiologix™ XF SR
Catalog number: 320
10 mL
$109.00

Physiologix™ XF SR
Catalog number: 322
100 mL
$1,092.00
Specifications
| Classification | Xeno-free, serum-free |
| Source | Transfusion-grade human material |
| Infectious Disease Screening | Not detected |
| Quality | cGMP* |
| Sterility | Sterile |
| Endotoxin | ≤ 0.25 EU/mL |
| Mycoplasma | Negative |
| pH | 6 to 8 |
| Shipping Conditions | Dry ice |
| Storage Temperature | ≤-80°C |
| Shelf Life | 2 years |
| Research Category | T cell research, stem cell research |
Documents and Downloads
Take a closer look at our helpful product information to learn how our cell culture media products and services can support and accelerate your groundbreaking research.
Physiologix™ Product Note
Review the technical details, features, and benefits of Physiologix xeno-free serum replacement.
Physiologix™ Performance on MSCs White Paper
Explore how to improve stem cell growth, expansion, and viability with Physiologix.
Instructions for Use
Review the Physiologix instructions for use to ensure proper use.
Need Help?
Get in touch with our customer support team for any questions you may have.
Visit NB-Lux™
View and order all available supplements and cell culture media products.
Need Cell Media?
Explore our portfolio of classical, specialized, and custom cell culture media.
Resources
Explore our educational resources to learn how Physiologix can be a competitive advantage on your path to commercialization.
FAQs
What is Physiologix™ XF SR?
Physiologix is a GMP, xeno-free media supplement made for stem cells and T cells that replaces fetal bovine serum (FBS) or human serum in cell culture media. Our serum replacement is extracted from transfusion-grade blood products.
What type of cells were grown using Physiologix™?
Physiologix has been used to grow a variety of immune and stem cells including HEK cells, mesenchymal stem cells (MSCs), T cells, and PBMCs.
Can Physiologix™ be delivered in bags?
What is the shelf life of Physiologix™?
Physiologix, as a standalone, is stable for 2 years when stored frozen at ≤–80°C.
Is a Drug Master File (DMF) available for Physiologix™?
When requested, Nucleus Biologics can provide an extensive technical data packet. A traditional DMF is not available at this time.